Photo courtesy of USC Viterbi
Assistant Professor Megan McCain has received a 2016 Scientist Development Grant from the American Heart Association.
The three-year, $231,000 grant supports highly promising early career scientists in cardiovascular and stroke research. McCain, Gabilan Assistant Professor of Biomedical Engineering and Stem Cell Biology & Regenerative Medicine, and her research team will focus specifically on developing human-based platforms to test drugs for heart failure caused by muscular dystrophy.
In the past, pharmaceutical companies and academic labs have used mice with genetic mutations to better understand the incurable disease. In USC Viterbi’s Laboratory for Living Systems Engineering, McCain, who was first attracted to studying the heart after seeing heart cells rhythmically contract on their own when grown in a dish, will use a more “human-relevant” engineering approach.
“Many times, aspects of diseases that are identified in mice don’t translate to humans, so mice are not ideal model systems,” McCain said. “Animal models are also quite expensive—they take a lot of time at a lot of cost.”
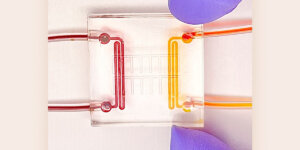
In lieu of mice, McCain’s team will utilize induced pluripotent stem cells derived from patients with muscular dystrophy. This new technology entails reprogramming human skin cells into stem cells that can then be turned into human heart cells. The heart cells will then be grown on scaffolds made from custom biomaterials that better mimic the human body.
“We take heart cells and grow them in an incubator, which people have been doing for a long time,” McCain said. “However, most times researchers grow cells on a plastic dish that doesn’t mimic the native human body very well. The cells lose their architecture and are grown on a substrate that doesn’t match the mechanical properties of the native heart.”
Once the new heart cells are fixed to the custom-engineered platforms, the team will study how strongly the cells contract in response to drugs and identify how the disease progresses.
“Pharmaceutical companies often screen drugs by seeing if the cells are alive or dead because they don’t have sophisticated tools for measuring contraction,” McCain said. “Another goal of our work is to develop these platforms to extract functional information instead of just toxicity because a lot of things can go wrong with your heart before it fails completely.”
This dynamic model, nicknamed “heart-on-a-chip,” employs a hydrogel scaffold that is tuned to mimic the stiffness of heart tissue, both healthy tissue that is soft and squishy and stiffer tissue meant to replicate a heart affected by muscular dystrophy, which has a lot of scarring.
From there, the team will test drugs and therapies that other model systems have shown could be promising to treat muscular dystrophy.
Published on September 26th, 2016
Last updated on March 10th, 2017