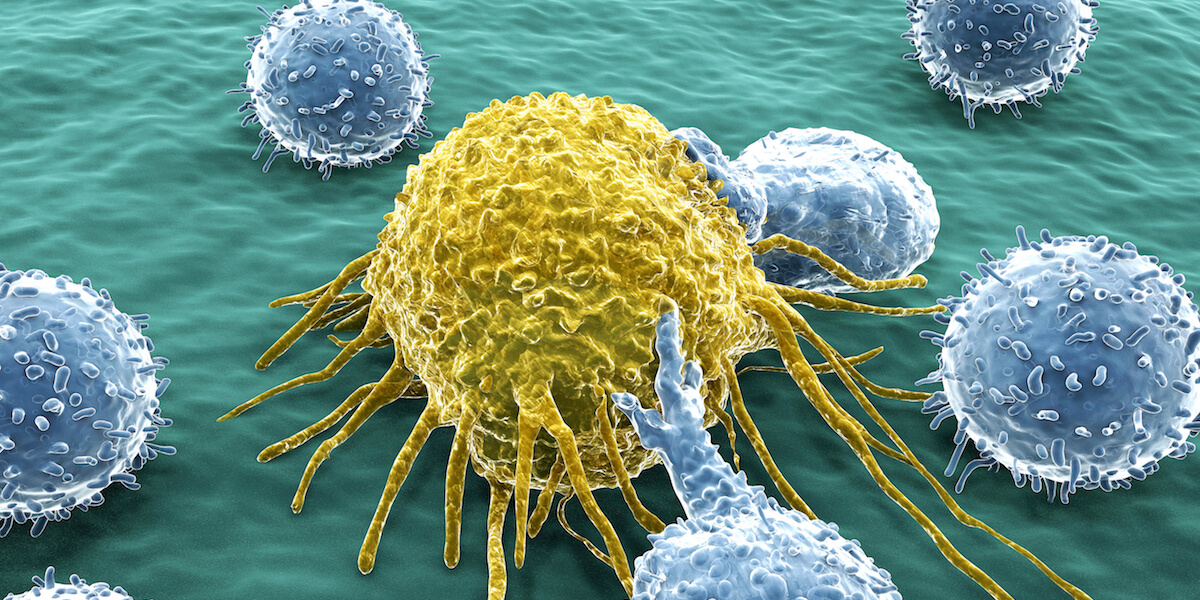
T-cells play an integral role in the body’s defense system. This picture shows activated T-cells attacking a tumor cell. Photo/Getty Images
While radiation and chemotherapy have been common cancer treatments for nearly a century, immunotherapy has gained traction in recent years as an alternative treatment.
And rather than use chemicals and radiation foreign to the body, immunotherapy harnesses the body’s own defenses to fight disease, a science that can be difficult to predict.
To that end, USC Viterbi’s own Stacey Finley, Gabilan Assistant Professor of Biomedical Engineering and head of the Computational Systems Biology Lab, is working to aid in the design of novel cancer immunotherapy using predictive models—a method of simulating real-life outcomes with math equations.
As one may remember from health class, T-cells are important immune cells that the body uses to fight foreign cells. In terms of battling cancer, T-cells attack tumor cells when triggered by molecules on the surface of the tumor cells, called antigens. A protein called LCK or lymphocyte-specific protein tyrosine kinase plays an important role in this process.
“We’re the first researchers to compile a series of predictive models that really look at LCK regulation in great mechanistic and molecular detail,” said Finley who collaborated with Professor Pin Wang, the Zohrab A. Kaprielian Fellow, and his ImmunoBioengineering and Bionanotechnology Lab and Assistant Professor Nicholas Graham whose research in the USC Mork Family Department of Chemical Engineering and Materials Science focuses on cancer systems biology.
Finley believes that a better understanding of how LCK is regulated can help researchers exploit its role in activating T-cells to fight cancer.

Stacey Finley receives her CMBE Journal Young Innovator Award from 2016 BMES Pritzker Award recipient Nicholas Peppas at the 2016 BMES Annual Meeting held October 5-8 in Minneapolis. Photo Courtesy of Viterbi Staff
LCK works by activating T-cell receptors called chimeric antigen receptors, or CARs for short. Generally speaking, receptors are proteins that span the cell membrane and bind other molecules like antigens, causing a biochemical reaction.
“The binding of the antigen to the CAR leads to an activation of a whole host of intracellular signaling leading to the T-cell,” Finley said. “That leads to the T-cell secreting certain cytotoxic factors, such as those which lead to death of the cancer cell or the T-cell proliferating and increasing in numbers to attack more cancer cells.”
Historically, LCK was thought to sometimes be completely inhibited at the molecular level, leaving it powerless to prod the T-cells to do their job. Finley and her team were able to use the math models to make novel predictions showing that is not the case—that LCK can still activate T-cells even when inhibited.
While Finley’s lab can mathematically model combinations of CARs, Wang’s lab can actually construct the CARs in order to conduct in vitro experiments which take place in live cell cultures.
“If we think more long term, as in what is the clinical application, this predictive modeling can help us anticipate what are the most effective combinations of CARs to activate T-cells,” said Finley. “Perhaps, we can use the models to help design more effective CARs to impact cancer immunotherapy.”
This approach, called adoptive T-cell therapy, would potentially use custom engineered T-cells in cancer treatment.
“If we were able to extract the T-cells of a cancer patient, we could engineer them with the effective CAR we help design,” Finley said. “And help them to expand by instigating proliferation and attack cancer cells when put back in the body.”
For her novel research and mathematical modeling, Finley was one of 11 early career researchers selected as a 2016 Young Innovator by Cellular and Molecular Bioengineering. Through her continued efforts, Finley hopes to make a lasting impact in the field of immunotherapy.
Published on October 19th, 2016
Last updated on March 10th, 2017