Mechanical engineering students Siena Applebaum and Zhenghong “Harris” Zhou working in the lab. Photo/Kayly Luong
The transportation industry produced 28.5 percent of the United States’ greenhouse gas emissions in 2016, more than any other economic sector that year. The majority of these emissions come from cars, trucks and aircraft. As such, for years, the industry has been working to reduce its emissions footprint by looking to other fuel sources outside of gasoline, diesel and jet fuel.
One of these new fuels is hydrogen, a clean-burning alternative with zero emissions. Compared to gasoline, which releases carbon dioxide when burned, hydrogen releases only water.
“Using hydrogen as a fuel is as environmentally friendly as a fuel can be,” said Paul Ronney, professor of aerospace and mechanical engineering. “It doesn’t contribute to global warming, apart from the potential for carbon dioxide emissions depending on the energy source used during the hydrogen production process.”
In a recent study by Ronney, mechanical engineering PhD student Zhenghong “Harris” Zhou, and mechanical engineering undergraduate student Siena Applebaum, the team uncovered how the unique characteristics of this alternative fuel affect engine efficiency. Their study takes a close look at the factors that affect the flame’s structure when using hydrogen fuel. Their findings could be used to design more efficient emission-free engines for car and air travel.
In contrast to cars powered by hydrogen fuel cells, which are expensive to manufacture and require an electric motor to convert electricity to power, hydrogen fuel can be used with only minor modifications in existing internal combustion engine commonly sported by gas-powered cars and aircraft.
To capture what occurs in this type of engine, the researchers designed an apparatus that simplifies the complex processes that occur inside an engine combustion chamber.
“What happens inside an internal combustion engine obviously is very complicated,” Ronney said. “So, we designed a very simple flow system that would mimic some of the key aspects of what happens inside the engine.”
They designed a combustor in which fuel and nitrogen (used to dilute the fuel) are injected from the top, and oxidizer and nitrogen are injected from the bottom. The two streams meet in the middle, mix together and burn.
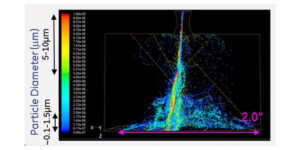
The team then watched how the transition region between the burning and non-burning regions, called the “edge-flame”, behaved – whether it advanced forward into the non-burning region or retreated back into the burning region and extinguished.
“Edge flames can be applied for making judgments that could help us to look at the flame instabilities in different situations,” said Zhou, lead author of the study.
To test different scenarios, they altered the flow rate at which the fuel and oxidizer are injected into the chamber and the amount of nitrogen diluent added to them.
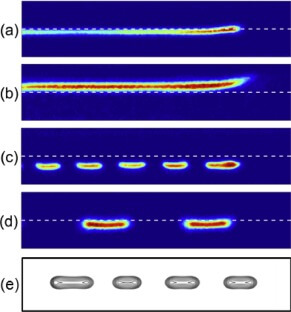
Observed edge-flames: a) advancing continuous flame, b) retreating continuous flame, c) advancing broken flame, d) stationary broken flame, and e) broken flame theorized by Thatcher and Dold. Image/Zhenghong “Harris” Zhou
The team quickly found that the unique properties of hydrogen led to a very interesting flame behavior that had only been theorized prior to their study: When more diluent was added to the fuel, the flame became unstable and a broken flame structure resembling a dashed line emerged.
Applebaum sees this strange behavior as a way of survival. “It enabled the flame to survive under conditions that they normally wouldn’t be able to survive,” she said.
“The flame is struggling so hard to just sustain the combustion,” added Zhou. “It’s a consequence of the properties of hydrogen.”
The broken flame is a result of hydrogen’s high diffusivity resulting from the small size and mass of hydrogen molecules, something no other fuels possess. Therefore, no other fuel could replicate this behavior. And perhaps it should be avoided. Ronney sees how this instability could hinder engine performance, as broken flames create regions of unburned fuel.
From their experiments, they were able to determine what ratio of fuel, oxidizer and diluent produced the strongest flames and which were most effective – another result that was a surprise.
“We show that under most conditions, with everything else being the same, you get stronger mixtures if you dilute the fuel side,” Ronney said. “It’s kind of counterintuitive because normally we burn air, which is highly diluted oxygen, with pure fuel. But here, this is saying, a better way to operate would be the opposite. Don’t dilute the oxygen, dilute the fuel.”
Their work earned themselves the Distinguished Paper Award of the Laminar Flames Colloquium of the 37th International Symposium on Combustion hosted by the Combustion Institute.
Published on April 2nd, 2019
Last updated on April 2nd, 2019