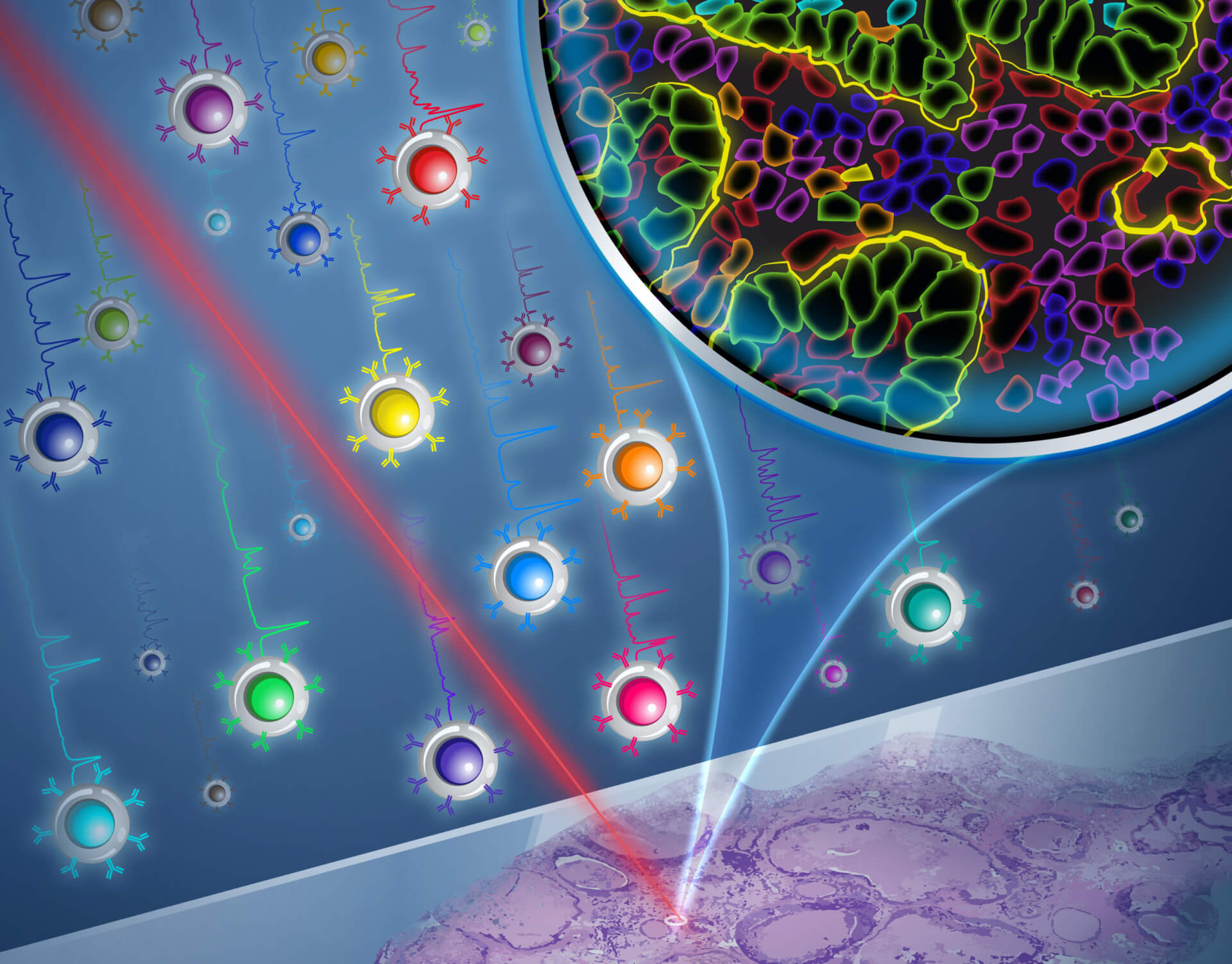
The Zavaleta Lab in USC Viterbi’s Department of Biomedical Engineering is harnessing nanoparticles in 26 different shades. The image shows how a Raman laser imaging technique can illuminate these particles to show the detailed molecular properties of cancer tissue samples. Image/Yekaterina Kadyshevskaya
Traditional medical imaging techniques such as X-rays can show us the structural outline of our bones and internal tissues – a silhouette in black and white. Now, a bold new generation of medical imaging aims to create a crystal-clear colorized picture in molecular-level detail, showing how cancer cells function and behave, what sort of proteins they express, and what the environment surrounding a tumor is like — crucial information that could change the game for discovering new drugs and personalizing medicine — choosing the most effective treatment for the individual patient.
Researchers in USC Viterbi School of Engineering’s Department of Biomedical Engineering have for the first time developed a molecular imaging method harnessing nanoparticles with cores made from gold, that can illuminate 26 unique biomarkers — features that show the precise behavior of cells. The research team, led by WiSE Gabilan Assistant Professor Cristina Zavaleta has been able to localize and unmix 26 unique nanoparticles, or what they call “flavors,” from one imaging pixel, which has applications for diagnosis of cancers, as well as neurological disorders, viral infections, and even non-medical applications in areas such as agriculture.
The work, co-authored by Postdoctoral Agilent Fellow Olga Eremina, Ph.D. student Alexander Czaja, Research Associate Augusta Fernando, undergraduate researcher Arjun Aron, and Postdoctoral Agilent Fellow Dmitry Eremin, has been published in ACS Nano.

WiSE Gabilan Assistant Professor Cristina Zavaleta
The research aims to provide information that can aid precision medicine and personalized treatments such as targeted therapies and immunotherapy, including treatments like CAR T-cell therapy, which harnesses and reprograms a patient’s immune system to effectively and directly target the unique features of cancer cells.
“The idea is to offer molecular information to physicians showing them what’s happening on the cellular level — For instance, if there’s something that cancer cells are expressing that is different to a normal cell,” Zavaleta said. “We’re trying to offer physicians new functional imaging tools, rather than just structural imaging tools.”
Zavaleta said the research community was starting to accept that one patient’s cancer or tumor can behave completely differently to another patient’s cancer. The unique proteins that cancer cells express, and the behavior of a patient’s immune cells in relation to the cancer cells, mean there could be vastly different treatments that can be more effective for different patients.
“Everyone has a unique molecular expression profile within their tumor; both within the tumor itself and in the tumor’s microenvironment — that is, what is around the tumor. It can be very different from patient to patient,” Zavaleta said.
Zavaleta said that currently when a tumor is removed or biopsied, pathologists use a white light microscope to interrogate the structural features of the tumor tissue.
“We want to offer physicians molecular information too, which is limited right now, in the sense that in most clinical settings you can only interrogate maybe a couple of biomarkers simultaneously,” Zavaleta said. “We’re excited to offer the ability to interrogate 26 biomarkers in a single imaging session without destroying the tissue, which is unprecedented right now.”

The imaging nanoparticles can target and highlight specific features of a cell in the same way that sprinkles can decorate a donut. Image/Sean Burkitt.
According to Zavaleta, nanoparticles are an ideal size for use as contrast agents — substances that interact with and illuminate cells and molecules for medical imaging. To imagine the scale of the process, Zavaleta said it was helpful to picture a 10-micrometer cell as equivalent to a 10cm donut, with a coating of 1mm colored sprinkles that can represent the different “flavors” of the around-100-nanometer particles targeting the unique aspects of the cell.
The imaging method the research team uses is known as Raman spectroscopy, which analyzes molecules by harnessing inelastically-scattered light — where the scattered photons have longer wavelengths. Using a laser, a Raman spectrum is acquired and measures the energy loss of the inelastically-scattered light, which in combination with the Zavaleta Lab’s engineered nanoparticles, creates a unique spectral barcode.
“So now my lab has been able to generate 26 of these barcodes, which has never been done before and pushes the limits of what Raman spectroscopy can do.
Zavaleta said that a key feature of the engineered nanoparticles is their gold center, which is crucial to generating a strong, clear signal during imaging.
“If the gold wasn’t there, this signal looks very noisy and is hard to detect, but there’s a plasmon resonance effect that happens in the presence of metallic features,” Zavaleta said. “When the Raman active layer in the molecule is in close proximity to gold, it generates this plasmon resonance effect, which gives us a very sharp, unique, and sensitive signal to detect,” Zavaleta said.
The new method is not currently intended for in vivo applications in human patients, given that gold nanoparticles have prolonged retention in the liver after intravenous injection. However, Zavaleta said that because most tumors are biopsied and resected, the imaging approach has promising applications for analyzing tissue samples in a way that can give physicians a precise molecular and spatial blueprint of a patient’s cancer. The method also offers the research community a powerful tool to examine existing banked tissue samples to better understand cancer behavior.
“What’s wonderful about being able to utilize this on tissue sections, is that we have tons of tissue from different patient types that has been preserved for years and is just sitting in tissue banks, that we can now retrospectively go back to and assess,” Zavaleta said.
“We’re really happy about being able to get the community excited about an entirely new imaging technique that can offer a colorized map of molecular expression across a patient’s tissue,” she said.
Zavaleta said the imaging technique wasn’t limited to cancer, but also had applications in imaging for neurological and infectious diseases, as well as non-medical applications in areas like food production.
“We’re not limited to just looking at human molecular targets. We can also go after plant-based cellular targets to understand why some crops are more drought tolerant or prone to different diseases,” Zavaleta said. “Food is so important to our way of life, and this could be a very useful tool in trying to create more efficient ways of farming.”
The Zavaleta Lab will now be exploring commercialization options for the new platform, while also further expanding the number of biomarkers they can illuminate in a cell sample beyond the existing 26.
Published on August 11th, 2022
Last updated on August 11th, 2022