 Engineers at Stanford University, Georgia Tech, USC Viterbi School of Engineering and the University of Tokyo have created a small, autonomous device with a stretchable/flexible sensor that can be adhered to the skin to measure the changing size of tumors. The non-invasive, battery-operated device is sensitive to one-hundredth of a millimeter (10 micrometers) and can beam results to a smartphone app wirelessly in real-time with the press of a button.
Engineers at Stanford University, Georgia Tech, USC Viterbi School of Engineering and the University of Tokyo have created a small, autonomous device with a stretchable/flexible sensor that can be adhered to the skin to measure the changing size of tumors. The non-invasive, battery-operated device is sensitive to one-hundredth of a millimeter (10 micrometers) and can beam results to a smartphone app wirelessly in real-time with the press of a button.
In practical terms, the researchers say, their device—dubbed FAST for “Flexible Autonomous Sensor measuring Tumors”—represents a wholly new, fast, inexpensive, hands-free, and accurate way to test the efficacy of cancer drugs. On a grander scale, it could lead to promising new directions in cancer treatment.
Each year researchers test thousands of potential cancer drugs on mice with subcutaneous tumors. Few make it to human patients, and the process for finding new therapies is slow because technologies for measuring tumor regression from drug treatment take weeks to read out a response. The inherent biological variation of tumors, the shortcomings of existing measuring approaches, and the relatively small sample sizes make drug screenings difficult and labor-intensive.
Yasser Khan, Assistant Professor of Electrical and Computer Engineering in the Ming Hsieh department of Electrical and Computer Engineering at USC, helped design this device as a post-doctoral researcher at Stanford.
“In some cases, the tumors under observation must be measured by hand with calipers,” says Alex Abramson, first author of the study and a recent post-doc in the lab of Zhenan Bao at the Stanford School of Engineering.
The use of metal pincer-like calipers to measure soft tissues is not ideal, and radiological approaches cannot deliver the sort of continuous data needed for real-time assessment. FAST can detect changes in tumor volume on the minute-timescale, while caliper and bioluminescence measurements often require weeks-long observation periods to read out changes in tumor size.
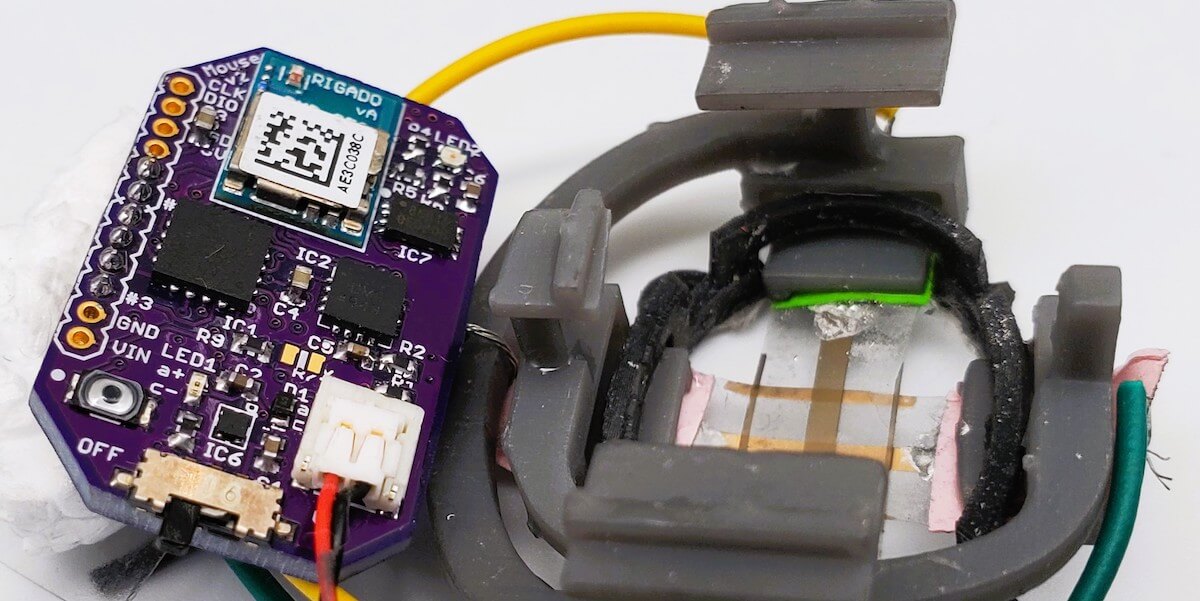
FAST’s sensor is composed of a flexible and stretchable skin-like polymer that includes an embedded layer of gold circuitry. This sensor is connected to a small electronic backpack designed by former post-docs and co-authors Khan and Naoji Matsuhisa, now at the University of Tokyo. The device measures the strain on the membrane—how much it stretches or shrinks—and transmits that data to a smartphone. Using the FAST backpack, potential therapies that are linked to tumor size regression can quickly and confidently be excluded as ineffective or fast-tracked for further study.
The researchers say that the new device offers at least three significant advances. First, it provides continuous monitoring, as the sensor is physically connected to the mouse and remains in place over the entire experimental period. Second, the flexible sensor enshrouds the tumor and is therefore able to measure shape changes that are difficult to discern with other methods. Third, FAST is both autonomous and non-invasive. It is connected to the skin, not unlike a band-aid, battery operated and connected wirelessly. The mouse is free to move unencumbered by the device or wires, and scientists do not need to actively handle the mice following sensor placement. FAST packs are also reusable, cost just $60 or so to assemble and can be attached to the mouse in minutes.
“This work is a prime example of how wearable electronics can further precision health technologies — we can monitor the growth of a tumor with tens of micron resolution using just a sensor and a cell phone app. We can observe the progression 24/7, unlike any of the existing imaging techniques, and precisely tell if a drug is working on not in treating the tumor ” said Khan.
The breakthrough is in FAST’s flexible electronic material. Coated on top of the skin-like polymer is a layer of gold, which, when stretched, develops small cracks that change the electrical conductivity of the material. Stretch the material and number of cracks increases, causing the electronic resistance in the sensor to increase as well. When the material contracts, the cracks come back into contact and conductivity improves.
Both Abramson and co-author Naoji Matsuhisa, an associate professor at the University of Tokyo, characterized how these crack propagation and exponential changes in conductivity can be mathematically equated with changes in dimension and volume.
One hurdle the researchers had to overcome was the concern that the sensor itself might compromise measurements by applying undue pressure to the tumor, effectively squeezing it. To circumvent that risk, they carefully matched the mechanical properties of the flexible material to skin itself to make the sensor as pliant and as supple as real skin.
“It is a deceptively simple design,” Abramson says, “But these inherent advantages should be very interesting to the pharmaceutical and oncological communities. FAST could significantly expedite, automate and lower the cost of the process of screening cancer therapies.”
Citation: Abramson et al., Sci. Adv. 8, eabn6550 (2022) DOI: 10.1126/sciadv.abn6550
Alex Abramson is now Assistant Professor of Chemical and Biomolecular Engineering at The Georgia Institute of Technology; Yasser Khan is Assistant Professor at the Ming Hsieh Department of Electrical and Computer Engineering at the University of Southern California; Carmel T. Chan is a former Senior Scientific Manager at Stanford University; Alana Mermin-Bunnell is a student at Stanford University; Naoji Matsuhisa is Associate Professor in the Institute of Industrial Science Department of Informatics and Electronics at the University of Tokyo; Robyn Fong is a Life Science Research Professor in the Cardiothoracic Surgery Department at Stanford University; Rohan Shad is a former Postdoctoral Fellow at Stanford University School of Medicine; William Hiesinger is Assistant Professor of Cardiothoracic Surgery at Stanford University; Parag Mallick is Associate Professor of Radiology at Stanford University; Zhenan Bao is the K.K. Lee Professor in Chemical Engineering at Stanford University.
The research was supported in part by an NIH F32 fellowship (Grant 1F32EB029787) and the Stanford Wearable Electronics Initiative (eWEAR).
The eWEAR-TCCI awards for science writing is a project commissioned by the Wearable Electronics Initiative (eWEAR) at Stanford University and made possible by funding through eWEAR industrial affiliates program member Shanda Group and the Tianqiao and Chrissy Chen Institute (TCCI®).
Published on September 17th, 2022
Last updated on November 15th, 2022