
Photo Courtesy of Jongseung Yoon
Although the sun provides a power unto itself, USC Viterbi’s own Jongseung Yoon has decided to take it one step further. Yoon has refined the complicated practice of solar water splitting, as detailed in a new paper he co-authored for Nature Energy.
“Using the sunlight, we can electrochemically split water into hydrogen and oxygen,” said Yoon, an assistant professor in the Mork Family Department of Chemical Engineering and Materials Science. “With a resource that is already abundant on earth, we can essentially make fuels from that, hydrogen. The idea itself is fancy, but the practical application has been seriously held up by issues in the materials.”
The journal Nature first reported on the concept of solar water splitting in 1972. However, Yoon’s paper, “Printed assemblies of GaAs photoelectrodes with decoupled optical and reactive interfaces for unassisted solar water splitting,” introduces techniques so novel that his research may be some of the very first to launch water splitting squarely in the path of achieving commercial viability within a decade.
The main procedural obstacles surmounted by Yoon’s group included enhancing stability, increasing efficiency and, most notably, cutting costs.
“Our group has been working on new ways to utilize III-V compound semiconductors for photocatalysis. Their excellent photophysical properties allowed us to achieve record-high efficiencies of converting solar energy into hydrogen, under 7 percent away from the 20 percent efficiency goal imposed by the U.S. Department of Energy,” said Yoon.
While past photoelectrodes only received light from one side, Yoon’s is bifacial. This invention completely diverges from the past few decades of water splitting techniques, which solely employed a single face to absorb light and drive catalytic reactions.
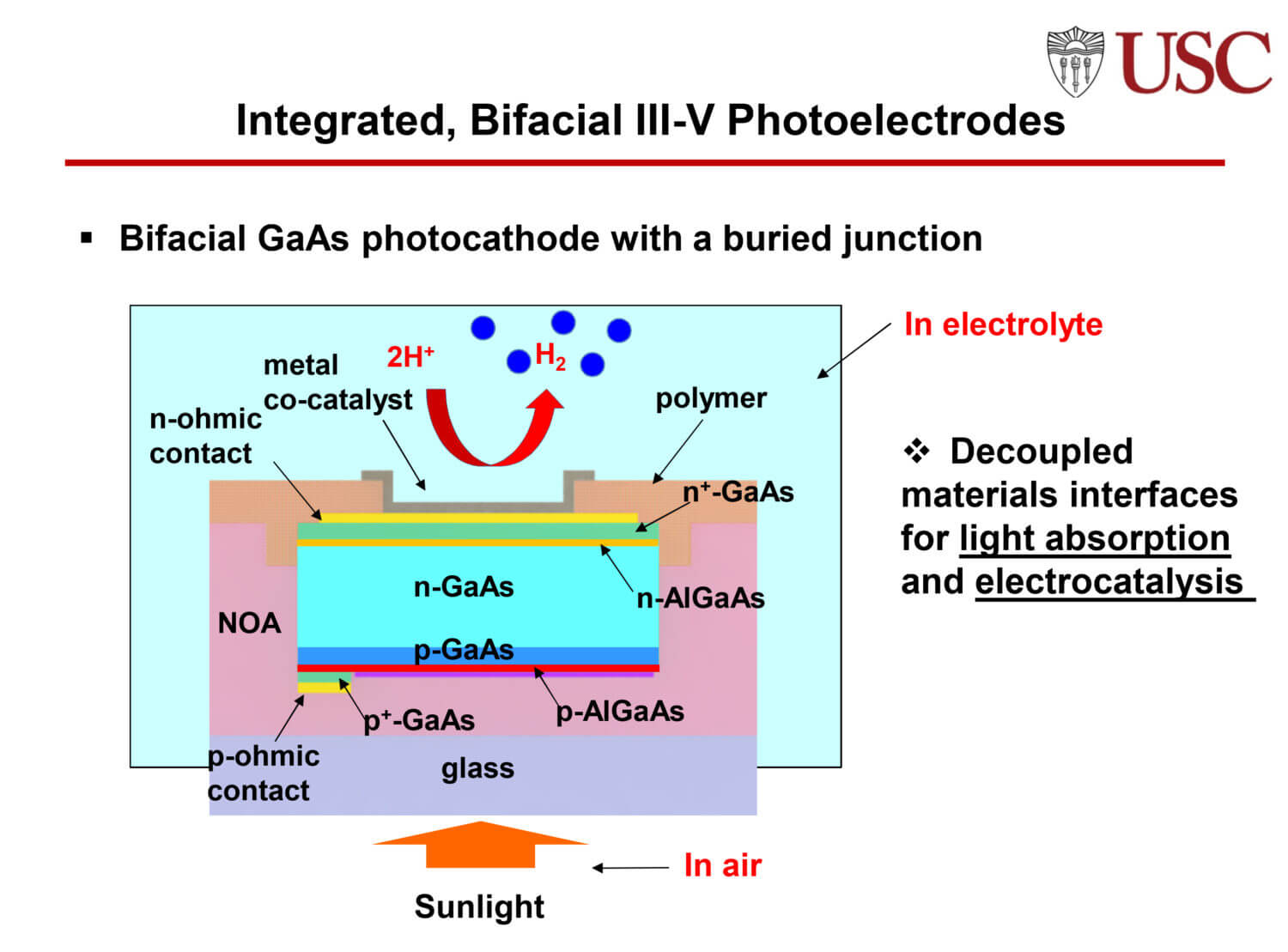 In addition, the substrate on which water splitting photoelectrodes are grown could only be used once in previous approaches. Yoon’s novel method allows the reuse of the expensive growth substrate .
In addition, the substrate on which water splitting photoelectrodes are grown could only be used once in previous approaches. Yoon’s novel method allows the reuse of the expensive growth substrate .
“Obviously, we’ve cut costs, but we’ve also managed to increase efficiency significantly,” said Yoon.
Other than the semiconductor itself, we also added a metal co-catalyst to make these reactions happen more effectively,” said Yoon. “This was really an essential component.”
However, a fundamental problem still remains at play, one that Yoon’s group has yet to completely overcome.
“Because all of these reactions happen at a single interface, there are too many conflicts between elements to fully optimize every function. For example, metals can reflect and scatter light, lowering the photoelectrode’s absorption and, thus, its efficiency,” said Yoon. “We could decide to use a thicker layer of metal to increase its catalytic capacity and stability, but that would impose a greater interference with light.”
This, in effect, is why Yoon’s bifacial photoelectrode invention is so vital.
“There are a lot of tradeoffs in a process like this,” said Yoon. “The window may be very narrow, but given the number of elements at play here, the combinations are abundant.”
As Yoon embarks on exploring the rest of this previously untapped potential, he poses a question: “If you combine hydrogen and oxygen, what happens?”
“They very vigorously react. Thermodynamically, that’s the favorable reaction. The opposite, making water into hydrogen and oxygen, is a completely uphill battle, but when it actually does occur, it’s one of the most powerful things to generate clean energy,” he explained. “And to think of how we could make this happen, it’s all natural, all just channeling the powers of the Earth.”
Published on March 29th, 2017
Last updated on March 29th, 2017










