Cardiovascular disease is the leading cause of death in the United States. Image/Pexels
Cardiovascular disease and kidney disease are two of the most urgent issues in global public health. In the United States alone, the U.S. Centers for Disease Control and Prevention reports that more than 1 in 7 adults is affected by chronic kidney disease, while cardiovascular disease remains our leading cause of death.
Biomedical engineers at USC Viterbi School of Engineering have now harnessed a naturally derived nanoparticle released from human cells to develop a powerful new gene therapy weapon against cardiovascular and kidney disease and many other potential therapeutic applications. The cardiovascular research has been published in Advanced Healthcare Materials, while the kidney disease-focused work is featured in Biomaterials.
The latest research comes from the laboratory of Eun Ji Chung, The Dr. Karl Jacob Jr. and Karl Jacob III Early Career Chair and assistant professor of biomedical engineering, chemical engineering and materials science. The Chung Lab conducts pioneering research in drug delivery and gene therapy, nanomedicine, and biomaterials. The lab has an extensive background in research involving the design and application of self-assembling synthetic nanoparticles for targeted drug delivery and treatments of diseases such as cancer, cardiovascular and kidney disease.
The lab is now focusing on novel applications of naturally derived versions of these nanoparticles, called extracellular vesicles (EVs). These EVs are membranous structures released from our cells. These non-synthetic particles that have been genetically engineered to enhance their therapeutic benefits, offer the potential for powerful new precision medicine applications that are well tolerated by patients.
“Our cells naturally produce these natural nanoparticles called extracellular vesicles,” Chung said. “So, we genetically engineered the cell to produce those EVs in a more therapeutic manner than what they would normally be secreting.”
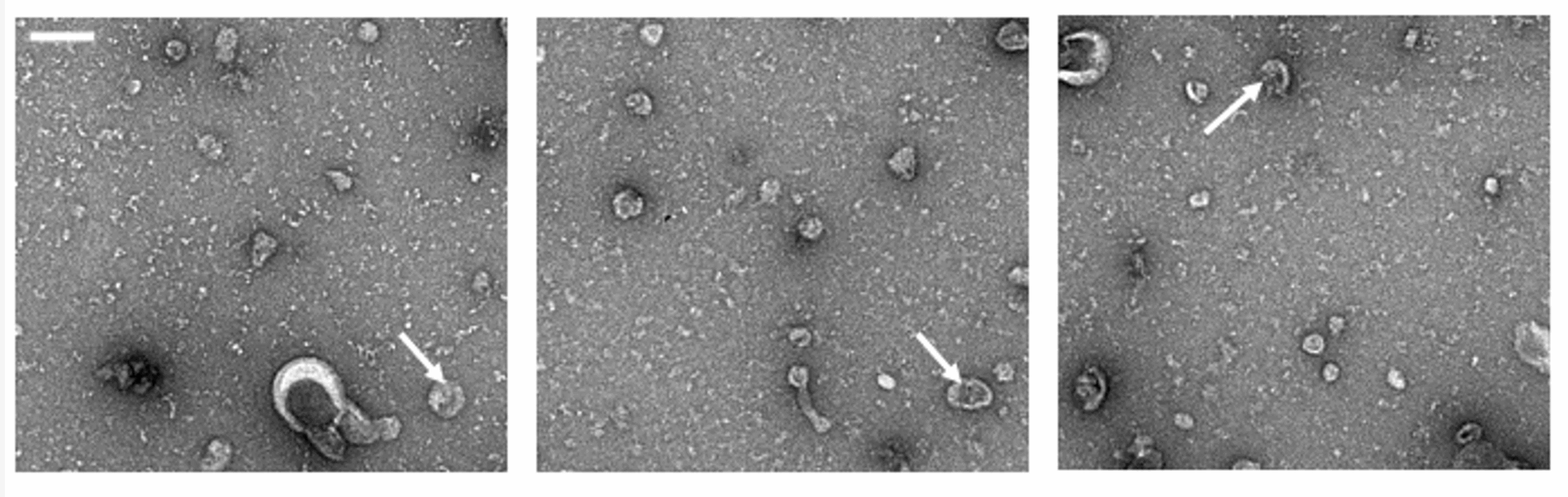
The Chung Lab’s extracellular vesicles (EVs) — naturally derived particles released from cells that have been genetically engineered to enhance their therapeutic benefits. Image/Chung Lab.
A next-gen treatment for heart disease that piggybacks on the human body’s own cell-to-cell delivery system
Atherosclerosis is a common inflammatory disease of the cardiovascular system. It causes plaque to build up and rupture in the arteries, which can lead to heart attacks and strokes. According to the National Institutes of Health, around 50% of Americans between ages 45 and 84 have atherosclerosis and don’t know it. When atherosclerosis occurs in coronary arteries, blockages due to plaque or calcification-induced ruptures can lead to a clot, cutting off blood flow to the heart, which is the cause of most heart attacks.
Chung and her team have a strong track record developing nanomedicine approaches to atherosclerosis, as an alternative to current treatments, which focus on lowering low-density lipoprotein cholesterol (LDL) using statins, a group of medications that inhibit the enzyme that helps produce cholesterol.

Dr. Karl Jacob Jr. and Karl Jacob III Early-Career Chair Eun Ji Chung. Photo courtesy of Viterbi Staff.
The lab’s previous work has focused on developing engineered micelles that can target calcification in arteries. The team switched their focus to EVs, which are usually produced by the body for cell-to-cell signaling. An EV particle is secreted by one cell and then absorbed by another. It’s like the body’s intercellular delivery system — a system that could potentially be harnessed for therapeutic purposes.
“One of the reasons we became interested in EVs is because they’re natural nanoparticles versus the lipid nanoparticles and micelles that we typically use. Those are synthetic, even though they have biomimetic and degradable components, whereas these EVs are natural nanoparticles that the cell is producing,” Chung said.
The research team’s platform harnesses the power of healthy cells, which, in turn, naturally secrete healthy particles such as RNA, proteins and extracellular vesicles. Chung noted that the EVs secreted by healthy cells will also have properties that help fight off atherosclerosis.
“But those properties are low level, so we engineer the cell to really boost it up,” Chung said. “Now, what they’re secreting performs thousands of times better in vivo.”
The Chung Lab’s therapy engineers the EVs to increase the naturally occurring microRNA 145, which targets vascular smooth muscle cells, which are responsible for the formation of plaque in the arteries.
“So, our hypothesis was, especially for chronic diseases like atherosclerosis, instead of dosing patients with synthetic nanoparticles, if we just dose them with natural nanoparticles, wouldn’t that be safer in the long run?” Chung said. “Because these patients are going to be dosed with therapies for the rest of their lives — that was the impetus in the beginning for us using the EVs. Because these EVs are innate and natural, they have this ability to be taken up by other cells.”
In a head-to-head comparison experiment between the Chung lab’s engineered EVs and synthetic nanoparticles, the research team found that the EVs were more effective at reducing plaque formation.
“We did a dose match, and the EVs have a much better potency. So not only are they natural, but something about their properties allows them to go inside the cell to a greater extent,” Chung said.
A New EV Toolbox to Tackle Kidney Disease
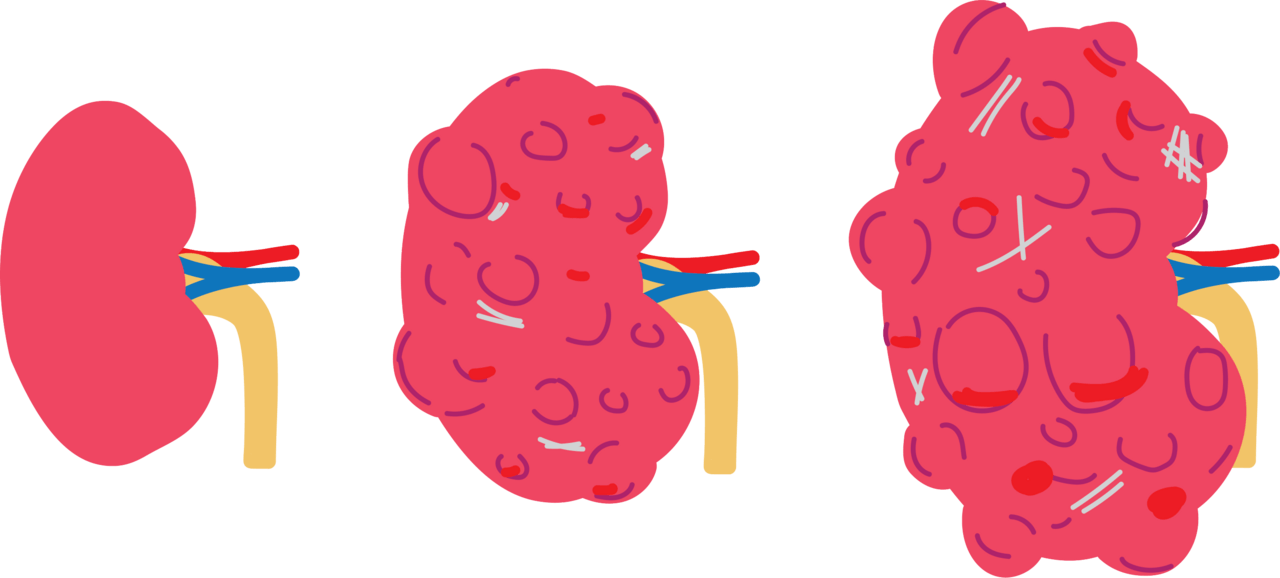
Left – right: A healthy kidney, a cystic kidney, and (far right) a kidney with numerous cysts, consistent with autosomal dominant polycystic kidney disease (ADPKD). Image/Wikimedia Commons.
Chronic kidney disease (CKD) is a widespread health issue affecting nearly 600 million people globally, often marked by a slow decline in kidney function. The Chung Lab has been researching new treatments for kidney disease using their specially engineered nanoparticles to ferry a payload of drug molecules to the kidney. The lab’s latest work looks at how EVs can instead be harnessed as a potential treatment for autosomal dominant polycystic kidney disease (ADPKD),
ADPKD is a common inherited form of kidney disease. It is caused by flaws in specific genes (PKD1 or PKD2), leading to numerous cysts growing in the kidneys, which can eventually cause kidney failure. Current treatments for ADPKD offer limited help. Drugs like Tolvaptan don’t target the kidneys directly and don’t fix the underlying genetic problem – the faulty proteins these genes produce. They often only slow the disease slightly, can have notable side effects, and don’t prevent the eventual need for dialysis or transplant for many patients.
Chung and her team are now investigating a safe and effective treatment by using urinary EVs secreted from healthy kidney cells that contain functional proteins that are missing in the EVs of ADPKD patients.
“In genetic kidney diseases like ADPKD, patients have a certain mutated version of a gene, and that gives either no protein or a dysfunctional protein. But if you get the EVs from a healthy donor, those EVs are loaded with the normal version of the protein,” Chung said.
In mouse studies, the team successfully demonstrated that less disease occurred, and cyst development was significantly slowed when healthy urinary EVs were harnessed to deliver their functional protein cargo directly to the diseased kidney cells. In addition, the treatment caused no adverse effects even after repeated doses.
Chung said that the latest work targeting both kidney disease and atherosclerosis showed that EVs hold a great deal of promise as an adaptable, naturally derived therapeutic tool to potentially treat a wide range of conditions.
“I feel like EVs really are the next wave of nanotherapeutics, like gene therapy, protein therapy and cell therapy. While our lab is still working on synthetic nanoparticles, around 50% of our workload is now in EV engineering, and all that work is coming to fruition,” Chung said.
Published on May 21st, 2025
Last updated on May 21st, 2025