Photo/Shutterstock
Through mathematical models and computer simulations, USC Viterbi researchers are finding ways to optimize chemotherapy schedules based on the composition and characteristics of cancer cells.
Using the biology-inspired evolutionary game theory, Professor Paul Newton and recent Ph.D. recipient Jeffrey West developed a tool that predicts how cancer cells will respond to different chemotherapy schedules and what may be the best course of treatment. Their method goes far beyond what previous mathematical models were capable of by applying evolutionary information and using cell-based data. And because they can run computer simulations based on their model, they are not subject to all of the restrictions associated with clinical trials.
In their latest study, published in the December issue of the journal Cancer Research, they determined that chemotherapeutic schedules should be designed based on the growth rate of the tumor. Specifically, cancers associated with fast-growing tumors, like brain cancer, should be treated using a low drug dose administered continuously, as opposed to a high drug dose given periodically.
“What we’re doing is trying to design chemotherapeutic schedules to better overcome some of the known problems associated with chemotherapy,” said Newton, professor of aerospace and mechanical engineering, mathematics and medicine — the latter at the Norris Comprehensive Cancer Center at the Keck School of Medicine.
A major one of these problems, which has become a focus of their research, is chemotherapeutic resistance by competitive release.
Traditionally, to reduce and potentially eliminate tumors, patients are given a chemotherapy drug and a schedule that targets all cancer cells. They receive this drug until the tumor is gone, most commonly through a high dose once every two or three weeks — also known as a maximum tolerated dose, or MTD, schedule — but sometimes through a low dose, continuous treatment plan known as low-dose metronomic, or LDM.
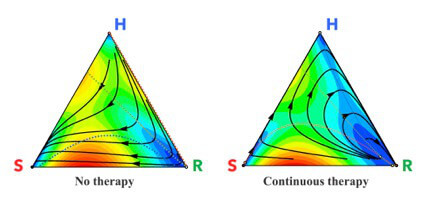
Three competing subpopulations of cells: healthy cells (H); sensitive cells (S); and resistant cells (R). When there is no chemotherapy (left), every initial state eventually evolves to the left sensitive corner signifying a fully formed tumor. With a continuous dose of chemotherapy (right), the sensitive cells are killed and the tumor initially shrinks, but eventually the resistant cells re-grow the tumor and chemotherapy is no longer effective. Illustration/Jeffrey West
However, what often occurs during these treatment plans is an initial shrinking of the tumor, indicating that they are seemingly on the correct path, followed by tumor regrowth despite the therapy they are receiving. The cause of this recurrence is due to the composition of the tumor itself.
“It’s not just one kind of cancer cell in your tumor,” Newton said. “It’s a whole bunch of different kinds of cancer cells that are all competing at different growth rates.”
After the chemotherapy drug has eliminated enough of the most prolific cancer cell types, the other cells that have survived are resistant to the drug and now have less competition for space and nutrients. These resistant cells have been “released from competition” and are now free to grow and divide at will. The next round of chemotherapy will therefore be far less effective.
“When you go in and take a biopsy or do imaging of the patient’s tumor, you can’t always count the number of resistant cells or tell which of those cells are going to be resistant,” West said. “One of the points of making a mathematical model is to predict things that can’t be directly monitored in the clinic.”
Their predictive tool allows them to test different chemotherapy plans on a tumor while accounting for the changing cell populations.
To do so, they used existing data on cancers treated by either MTD or LDM and modeled the competition between cells using evolutionary game theory, a mathematical framework that uses Charles Darwin’s concept of “survival of the fittest.” The model picks two cells at random for a head-to-head competition where the winner is determined based on their “fitness,” which is dependent on the cell type’s growth rate.
The players are the healthy cells, the targeted cancer cells and the resistant cancer cells and the game is played over and over again in the computer simulation as the different populations of cells compete. For each treatment scenario, the model hosts millions of competitions to simulate how the tumor will most likely progress over time.
“We cycled through all possible combinations of scheduling and different drug concentrations and we sort of created a histogram of what would be the most likely benefits in different scenarios,” Newton said.
They found that chemotherapeutic schedules should be determined by the growth rate of the tumor, something not currently done in a clinical setting. For example, fast-growing tumors, typical with brain cancer, are better controlled using LDM, while MTD worked better against slow-growing tumors, typical with prostate cancer.
In addition to comparing existing therapies, they will use their model to develop treatment plans that adapt as the tumor changes. This requires monitoring the different cell populations that make up the growing tumor and adjusting the schedule based on the data. In engineering circles, this approach is called “closed-loop” control.
“The hope for the future is to have patient specific models where a patient walks into a clinic and you can type in their information and then run the model… and predict the optimal schedule and drugs,” West said.
After completing his Ph.D. in mechanical engineering in August, West is now working as a post-doctoral researcher for the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Fla. Working in their Integrative Mathematical Oncology Department, he is using the mathematical model to test treatment plans being developed by clinicians at the center before they are applied to patients during clinical trials.
“The way that new clinical trials are designed is in large part based on the intuition of the clinician, but the timelines of these things can be years to even decades,” West said. “Math models can sort of close the loop and test the clinician’s intuition in mathematical abstraction before the clinical trial even starts.”
Published on March 27th, 2018
Last updated on April 4th, 2018