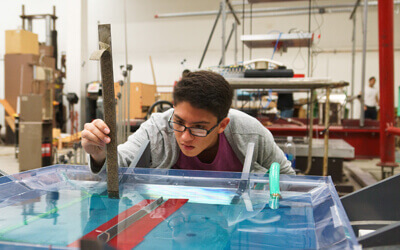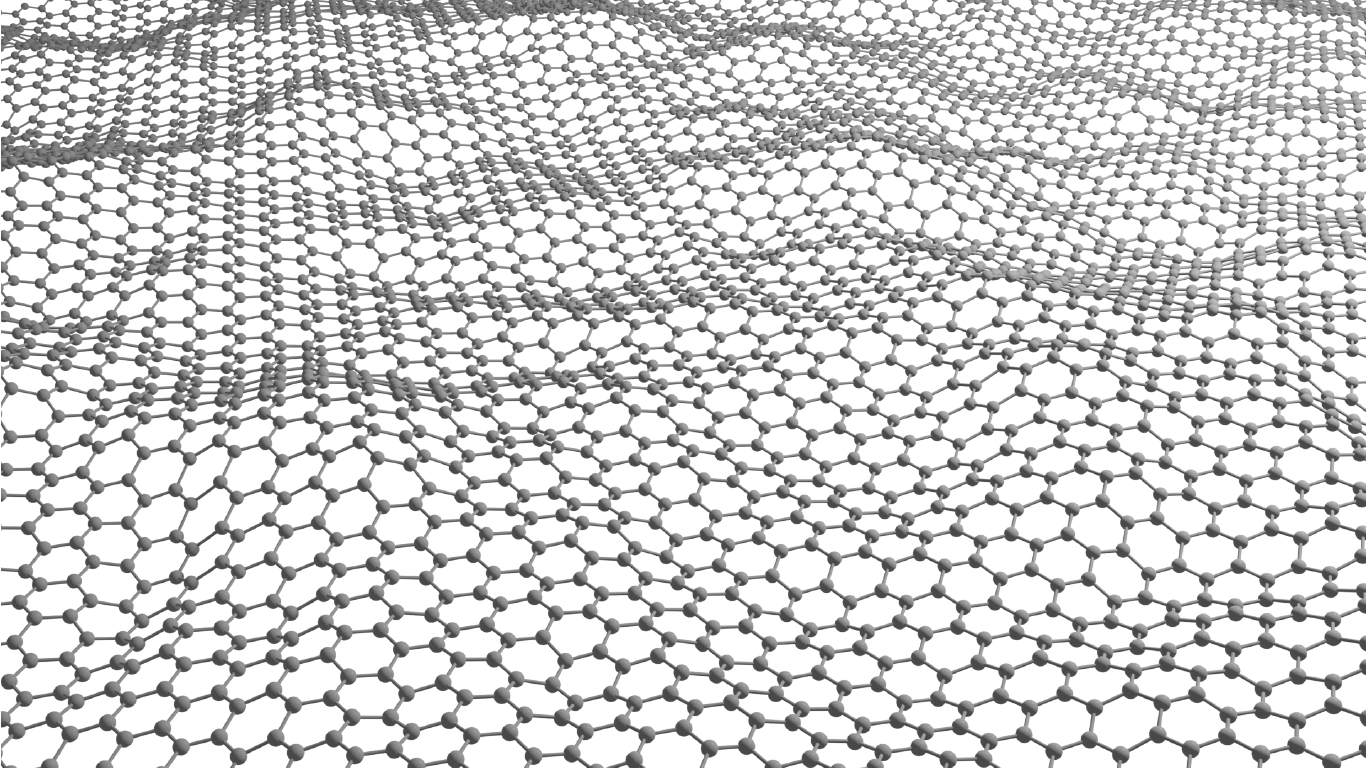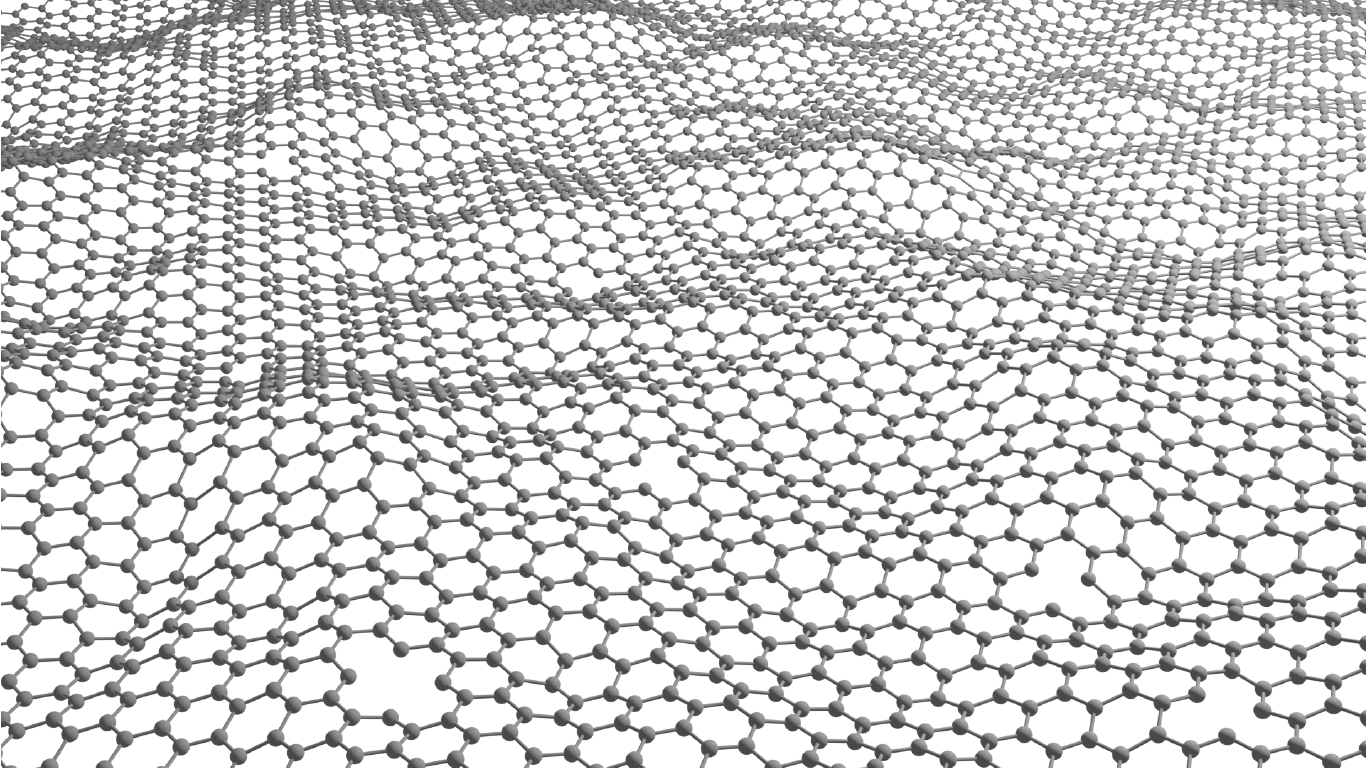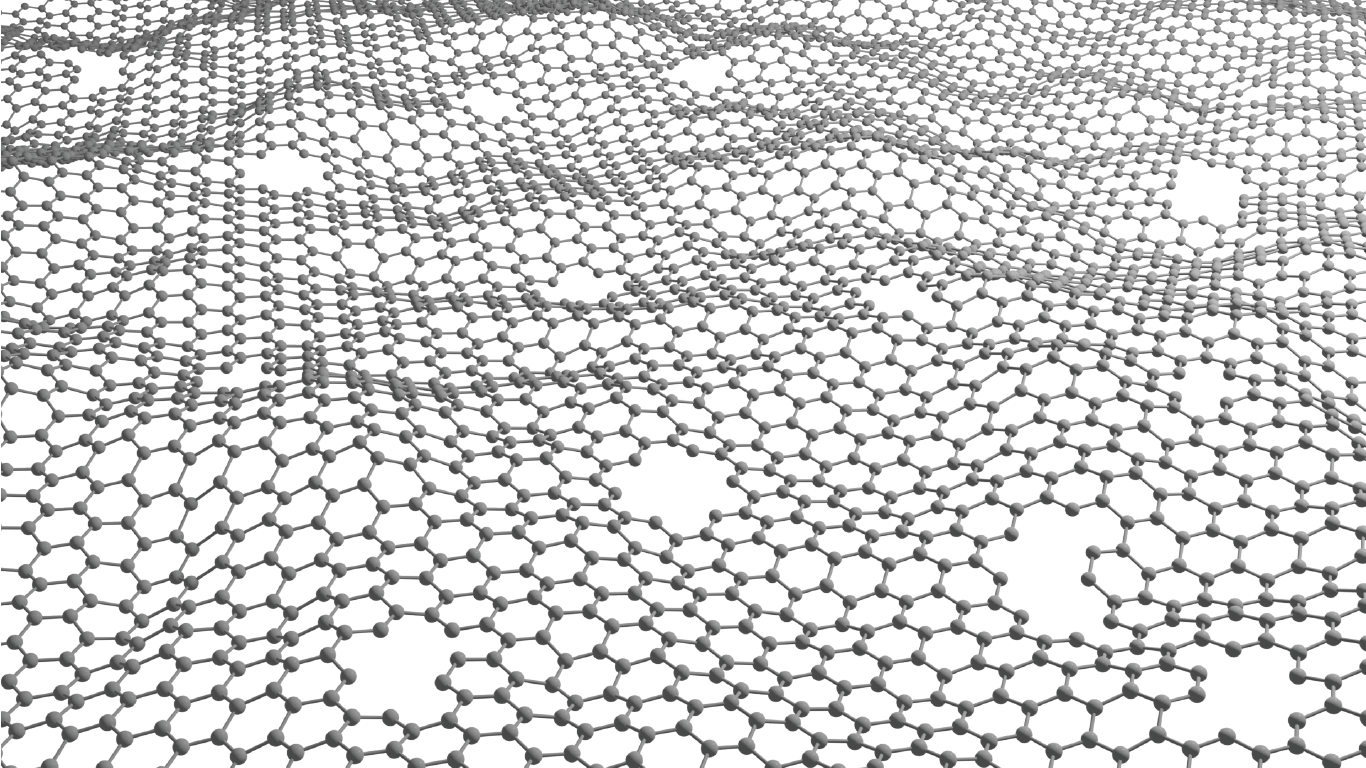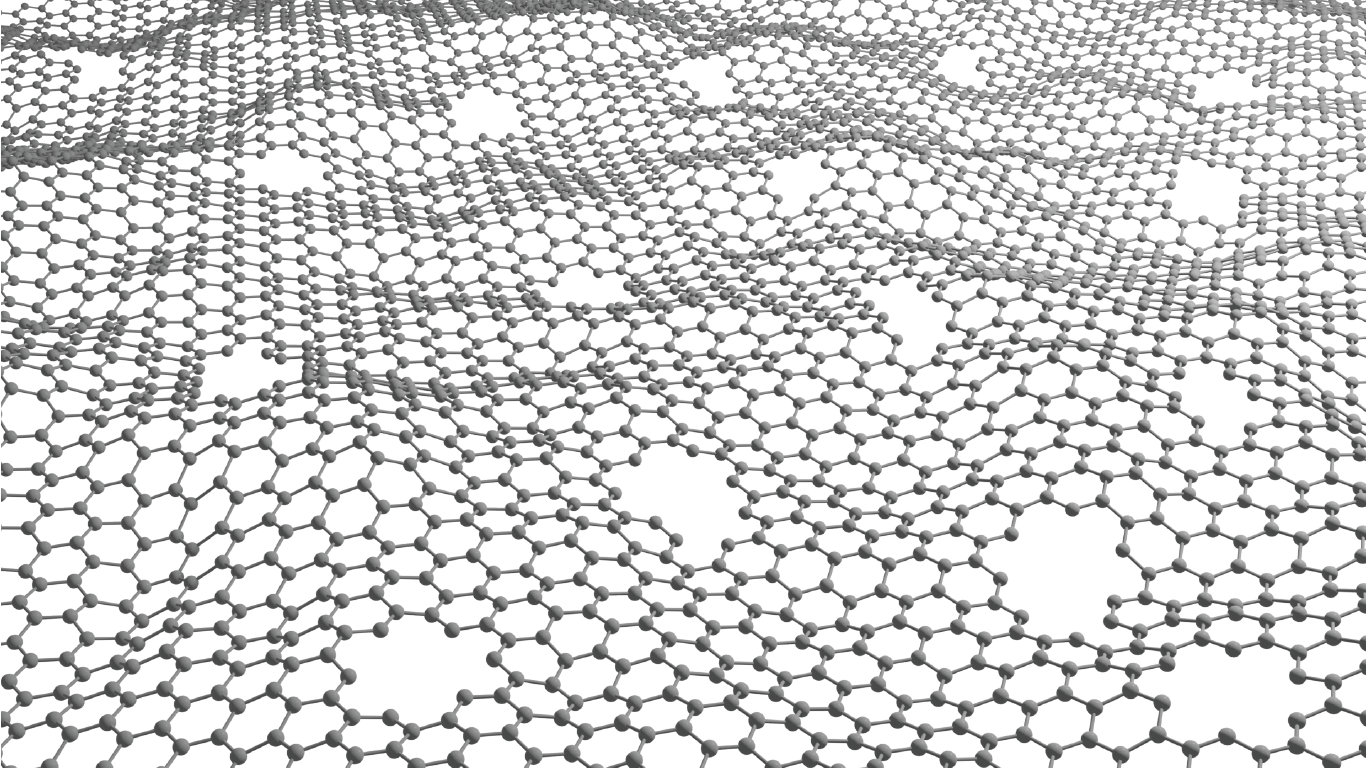
Luis Francisco Villalobos is creating atom-thick nanoporous molecular filters by etching pores into a layer of graphene lattice – a super-thin layer of carbon with a precise hexagonal structure. Image/Joseph G. Manion and Luis Francisco Villalobos.
Separation technology is something many of us need just to face the day. Each morning, our trusty coffee filter works its magic, separating the coffee grinds and converting water into that bracing dose of liquid energy we require to function.
Luis Francisco Villalobos is interested in how this process can be harnessed at an atomic scale using advanced membranes made from energy-efficient materials. These cutting-edge membranes can secure our drinkable water supply, capture valuable elements from waste products, and aid the transition to clean energy.
In January, he joins the Mork Family Department of Chemical Engineering and Materials Science as an assistant professor, boosting the department’s capacity in separation technology to aid water filtration and pollution mitigation techniques such as carbon capture.
Villalobos said that half of the energy expended by the chemical industry goes into the industrial process of separation.
“My personal view is that this number will only increase because critical materials — like water, lithium, nickel and other metals — are stressed to levels where we need better separation technologies, so we can maximize their recovery,” he said.
Villalobos hopes to reverse this carbon-intensive trend by developing super-thin membranes with highly controlled material properties to create an energy-efficient and modular separation process.
“It’s like a coffee filter – the filter allows the water to pass through but retains the coffee grains. This is a separation that we can see. But the separation that I’m targeting is at the molecular level — separating one molecule from another in a stream,” Villalobos said.
One of the most common industrial applications of molecular-scale separation is the reverse osmosis membrane used for water desalination and filtration. Villalobos is developing technology for this application, as well as carbon capture and storage, and the recovery of valuable materials from waste liquids such as brine, mine tailings, and produced water — the naturally occurring saline water that comes out of the earth as a waste product from oil and gas production.
“We’re taking a waste stream and extracting something valuable out of it, so we can enhance the availability of these resources that are becoming increasingly scarce with time,” Villalobos said.
One key example of a product available in waste streams is lithium — an increasingly important material for electronics and battery storage. Lithium is abundant in the ocean and yet very difficult to extract in an energy-efficient way.
“We need to find better ways to extract lithium from known sources, but also find other sources where traditionally we don’t extract it,” Villalobos said. “Produced water comes out of oil reservoirs, and they need to do something with it. As it passes through rocks in the earth, it comes with a lot of metals in it, lithium included. So that’s one potential source.”
Etching holes into membranes as thin as an atom

Assistant Professor in the Mork Family Department of Chemical Engineering and Materials Science Luis Francisco Villalobos.
The cutting-edge membrane technology Villalobos is developing is known as an atom-thick nanoporous molecular filter. He works with graphene, a layer of carbon the thickness of a single atom, perfectly arranged in a hexagonal pattern. A pristine graphene layer has a very tight atomic structure that doesn’t allow molecules to pass through.
“But if we manage to drill a lot of precise holes on these atom-thick materials, then we can make very energy-efficient membranes,” Villalobos said. “Typically, the energy we need to spend to move molecules from one side of a membrane to the other is proportional to the thickness of our membrane. These materials are as thin as it gets.”
The pores can be etched by exposing the graphene lattice to an agent – usually, a molecule based on oxygen, such as ozone. This agent reacts with the graphene lattice to etch a nanopore.
Villalobos said that atom-thick nanopore membranes are attractive for research and industry because graphene is a very stable material that can withstand harsh chemical environments and can tackle various separation challenges.
“Because we control the pores, we can do a certain etching for a certain separation,” Villalobos said. “For example, if we want to recover lithium from brine, we can etch nanopores that allow lithium to pass but reject the other molecules present in the stream.”
Villalobos will investigate how to improve the effectiveness of nanopore etching in the graphene membrane by varying the ozone agent’s concentration while controlling the material’s temperature. The aim is to drill membrane pores at even higher densities, with better control over pore size.
Villalobos said he has always been a curious person. He was initially inspired to explore the wonders of membrane separation when he first saw how reverse osmosis systems can convert salt water to drinkable fresh water.
“Even now, we don’t fully understand why they work so well — why they manage to allow water to pass, and they reject all the ions present in the seawater,” Villalobos said. “When I saw this example in one of my classes during my master’s degree, I was fascinated about how to create these filters that can separate molecules. From there, it’s history. I have been working with membranes for more than ten years now.”
Forging new collaborations and mentorship opportunities
Villalobos comes to USC Viterbi from Yale School of Engineering and Applied Science, where he was a Swiss National Science Foundation postdoctoral research fellow. Before this, he was a postdoctoral research fellow at École Polytechnique Fédérale de Lausanne in Switzerland. He received his Ph.D. and M.S. in chemical engineering from King Abdullah University of Science and Technology in Saudi Arabia and his B.S. from Universidad Nacional Autónoma de México.
Villalobos will soon establish his research lab in the Mork Family Department, where he will also teach Introduction to Separation Processes. Villalobos said hiring and mentoring his first batch of research students will be a key highlight.
“One thing that attracts me a lot to the department is the fact that they combine materials science with chemical engineering because what I propose to do really sits between these two areas,” Villalobos said.
“I love to do research with a reason. I’m passionate about the applications that we are targeting to make a difference and decrease the amount of energy we spend on separations. I’m really happy that I’ll be able to collaborate with experts in both areas to push forward the development of the next-generation membranes that can tackle these challenges,” Villalobos said.
Published on August 31st, 2023
Last updated on August 31st, 2023