PhD students Regib Ahsan (Right) and Hyun Uk Chae with some of the devices used to accomplish this feat. (PHOTO CREDIT: USC Viterbi)
Electrons are lazy little things. They’re everywhere; embedded in literally every piece of matter in the universe. The problem is that they don’t like to be disturbed. Electrons are happiest when they’re at their lowest energy state; chilling out together beneath the surface of whatever material they find themselves in. This is a challenge for engineers, who need electrons for important devices related to imaging, health, defense, and more. Electron microscopes, high-power radio frequency amplifiers, and large particle accelerators are all examples where our ability to controllably emit electrons are critical. Unfortunately, getting these sub-atomic squatters up and moving often requires high-power, inefficient processes.
Enter Rehan Kapadia, assistant professor in the Ming Hsieh Department of Electrical and Computer Engineering. Kapadia and his students have been able to show that under certain conditions photons can be used to emit electrons more efficiently than previously demonstrated. Like, really efficiently. Like, ten to the power of five times more efficient. The team’s paper was published last week in Nature Photonics – one of the most prestigious photonics journals. So how did they do it and what does it mean?
First, they combined two different electron emission techniques together. Then, they used those two techniques on the best possible material. Finally, they optimized that material for maximum light absorption.
Before the question can be answered, we have to understand how electron emission works in the first place. Electrons can be extracted in several ways. You can heat the material they’re in (thermionic emission) or shine a light on the electrons to give them energy (photo emission). In both these cases the electrons are energized and forced up to the top of the material workfunction barrier where they can flow out.
The other strategy, field emission, involves applying an electric field to the material. Part of the reason electrons stay put is because the energy barrier is too wide. “By applying an electric field, you can actually shorten the material’s barrier width– leaking the electron wavefunctions out of the material,” said Ragib Ahsan, one of the students working on the project.
What happens next is really mind-blowing. The moment the electrons sense the barrier isn’t infinitely thick, they flow out. This time, they don’t pass over the top of the material, though. They simply tunnel right through it. Once electrons sense that the barrier has an endpoint, they are suddenly able to unlock the ability within themselves to flow through it. It’s like self-actualization on a sub-atomic scale.
It’s like self-actualization on a sub-atomic scale.
This brings us to Kapadia’s recent work. The problem for researchers isn’t getting electrons out; it’s getting enough electrons out without using powerful lasers which make devices bulky and limit where they can be used. Researchers have tried using low-energy photons, which can be easily generated, to emit electrons. The problem is that lower-energy photons don’t have enough power to knock an electron out of the material on their own. To solve this, Kapadia and his team combined the photo emission and field emission processes. The strategy is basically one of meeting in the middle: First, a lower-energy photon comes in and partially excites an electron. The low-energy photon has just enough juice to get the electron energized and moving up towards the material barrier. As this partially excited electron travels up, the field emission process kicks in, lowering the material barrier. Using these techniques in tandem, the electron is able to tunnel out through the barrier.
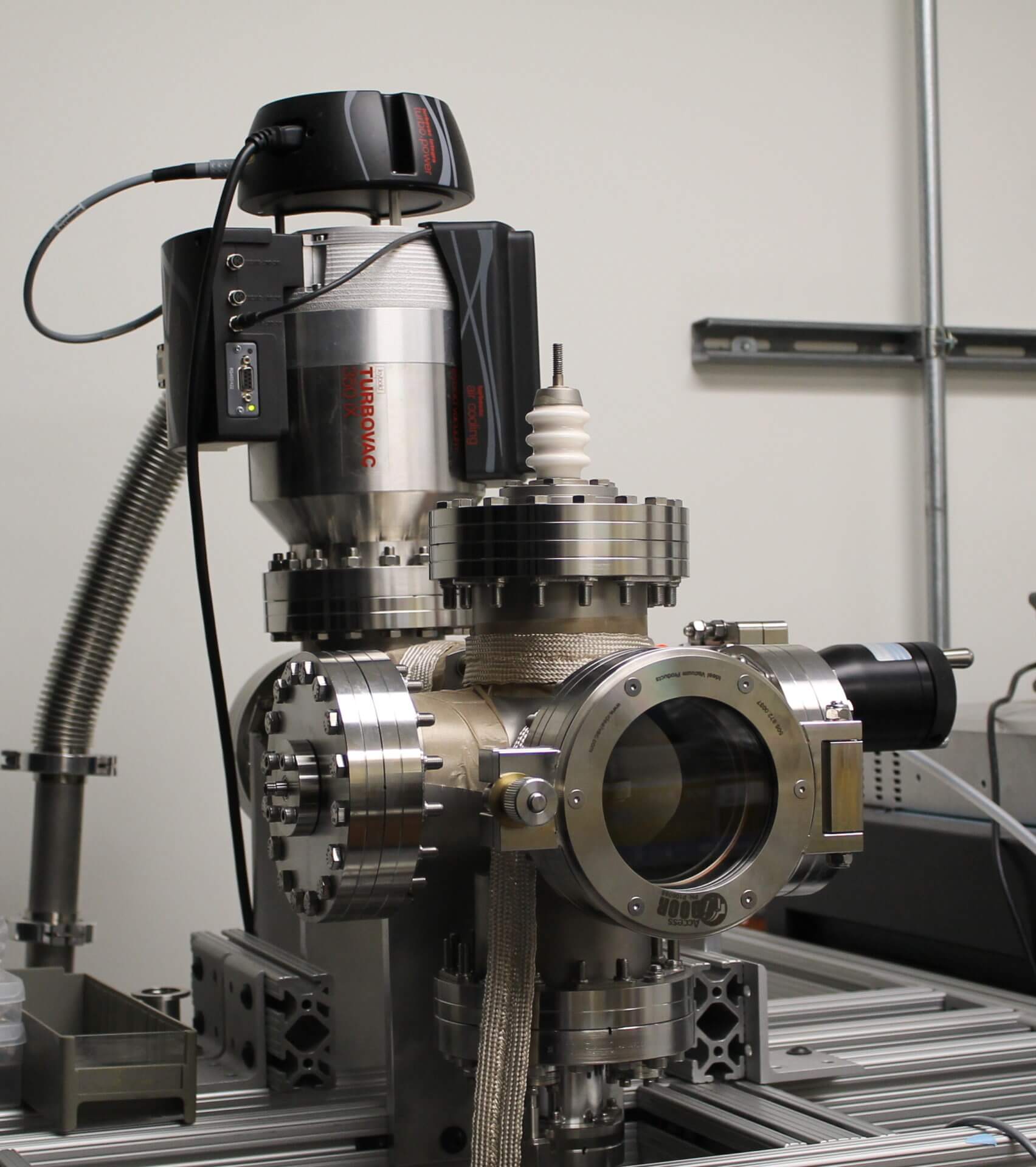
The graphene is placed in this vacuum device, designed and built in Kapadia’s lab, to help ensure maximum electron emission efficiency. (PHOTO CREDIT: USC Viterbi)
But there is still another inefficiency to solve for. As the excited electrons travel towards the surface, they lose some of their energy. Kapadia’s group solved that problem as well. This time, the solution was using a better material to emit the electrons from. Graphene is thinnest material in the universe – made of carbon and only one atom in thickness. With graphene, every excited electron is already at the surface, eliminating the loss of energy caused by electrons having to travel through a thicker material.
But wait, there is still yet another inefficiency to solve for! You see, graphene only absorbs about two percent of the light that falls on it and, if you’ve been paying attention, that light is needed to energize the electrons. This time, instead of shining light directly on the graphene, the group put the graphene on an optical waveguide – a structure that helps guide waves more effectively. This seemingly small change allowed more than ninety-nine percent of the light to be absorbed in the graphene.
So, to summarize, Kapadia and his team did three things to achieve this milestone. First, they combined two different electron emission techniques together. Then, they used those two techniques on the best possible material. And finally, they optimized that material for maximum light absorption. Then, and only then, were they able to accomplish this feat.
While Kapadia and his students have proven that they can take electron emissions to the next level, there is still more work to do. “The current devices are a nice proof of concept but taking them from the initial demonstration to a device that can be used in real-world applications requires more optimization. Now we have a roadmap for that and are going to see how far we can push this technology,” said Kapadia.
Published on October 7th, 2019
Last updated on April 8th, 2021