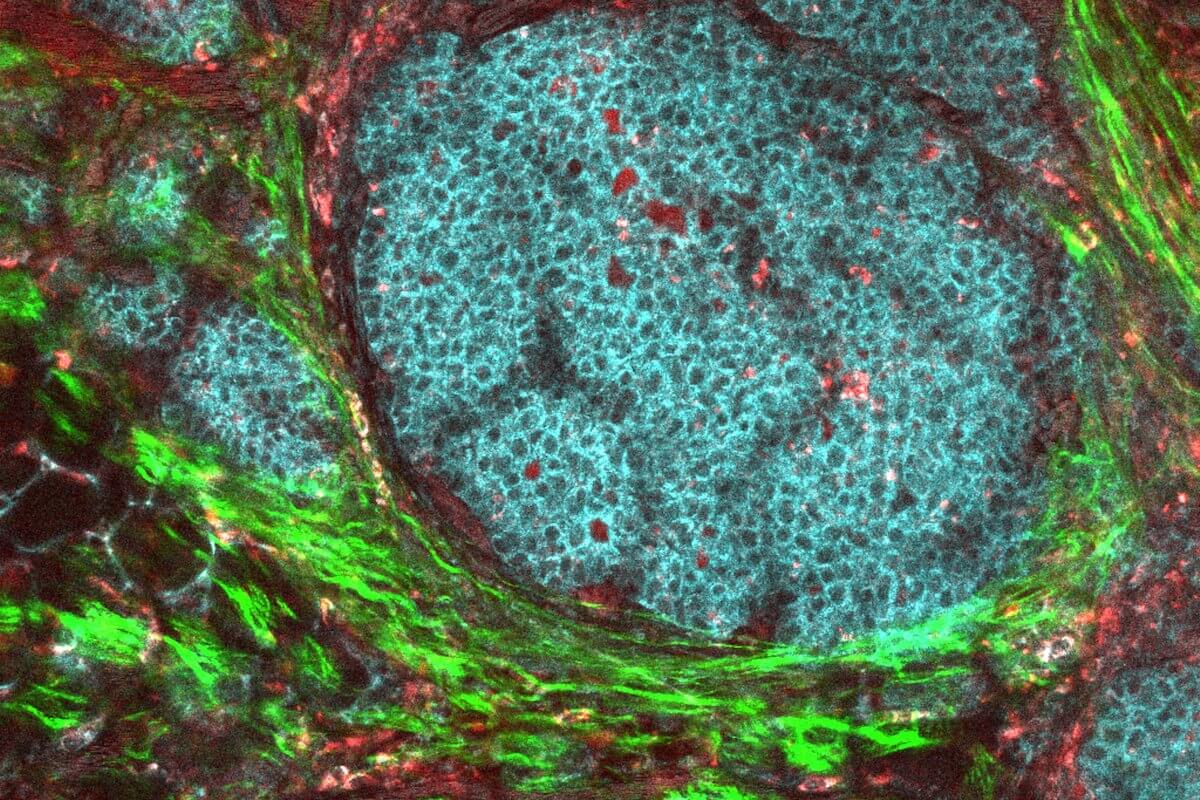
A breast cancer tumor and its surrounding microenvironment. Image/National Institutes of Health
Tumor cells are “cunning,” according to Peter Yingxiao Wang. They have a nefarious way of evading the human immune responses that fight back against these cancerous invaders. Tumor cells express programmed death-ligand 1 (PD-L1) molecules, which work like a defensive shield that suppresses our immune cells, posing a hurdle for targeted cancer immunotherapies.
Wang — the Chair of the Alfred E. Mann Department of Biomedical Engineering and the Dwight C. and Hildagarde E. Baum Chair in Biomedical Engineering — leads a lab that conducts pioneering research into engineered immunotherapies that harness the human immune system to create a future arsenal in the fight against cancer.
Wang Lab researchers have now developed a new approach that turns a tumor cell’s cunning defense mechanisms against it, converting these “shield” molecules into a target for the Wang Lab’s engineered Chimeric Antigen Receptor (CAR) T-cells that are programmed to attack cancer.
The work — led by postdoctoral researcher in the Wang Lab, Linshan Zhu, along with Wang, research assistant professor Longwei Liu, and their collaborators — has been published in ACS Nano.
CAR T-cell therapy is a revolutionary cancer treatment in which T-cells — a type of white blood cell — are removed from a patient and given the unique chimeric antigen receptor (CAR). The CAR binds to cancer cell-associated antigens, directing T-cells to destroy the cancer cells.

Dwight C. and Hildagarde E. Baum Chair in Biomedical Engineering Peter Yingxiao Wang. Image/David Baillot, UC San Diego Jacobs School of Engineering.
The Wang Lab’s latest work is an engineered monobody for the CAR T-cells that the team has labeled the PDbody, which binds to the PD-L1 protein defense molecule on a cancer cell, allowing the CAR to recognize the tumor cell and block its defenses.
“Imagine the CAR is a real car. You have the engine and the gas. But you also have a brake. Essentially, the engine and gas push the CAR T to move forward and kill the tumor. But the PD-L1 works like the brake that stops it,” Wang said.
In this work, Zhu, Liu, Wang and the team engineered the T-cells to block this inhibitory “brake” mechanism and convert the “brake” molecule PD-L1 into a killing target.
“This chimeric PDbody-CAR molecule can lead the CAR T we designed to start to attack, recognize, and clear the tumor. At the same time, it will block and prevent the tumor cell from stopping the CAR-T’s attack. In that way, our CAR T will be more potent,” Wang said.
CAR T-cell therapy is most effective in “liquid” cancers such as leukemia. A challenge for researchers has been to develop advanced CAR T-cells capable of distinguishing cancerous cells from healthy cells.
The Wang lab is exploring ways to harness the technology in a targeted way for tumors so that the CAR T-cells activate at the tumor site without impacting healthy tissue. In this work, the team focused on a very invasive form of breast cancer that expresses the PD-L1 protein. However, PD-L1 is also expressed by other types of cells. Hence, the researchers looked at a tumor’s unique microenvironment — the cells and matrices immediately surrounding a tumor — to ensure that their engineered PDbody would bind more specifically to cancerous cells.

Wang Lab Postdoctoral Researcher Linshan Zhu/Image: Praopim Limsakul
“We know that the pH is relatively low in the tumor microenvironment — it is a little acidic,” Zhu said. “So, we wanted our PDbody to have a better binding ability in an acidic microenvironment, which will help our PDbody distinguish the tumor cells from other surrounding cells.”
To enhance the precision of the treatment, the team harnessed an engineered genetic “gate” system called SynNotch that ensures the PDbody CAR T-cells only attack cancer cells expressing another protein known as CD19, reducing the risk of harming healthy cells.
“In simple terms, the T-cells will only be activated at the tumor site because of this SynNotch gating system,” Zhu said. “Not only is the pH more acidic, but also the tumor cell’s surface will determine whether the T-cell will be activated, giving us two layers of control.
Zhu said that the team used a mouse model, with the results showing that the SynNotch gate system guided the PDbody CAR T-cells to activate only at the tumor site, killing tumor cells while remaining safe to the other parts of the animal.
An evolution-inspired process for building the PDbody
The team harnessed computation and drew inspiration from the process of evolution to craft their specialized PDbody. Directed evolution is a process used in biomedical engineering to mimic the process of natural selection in a laboratory setting. The researchers created a directed evolution platform with a gigantic library of iterations of their engineered protein to discover which version could be most effective.
![PROFESSOR LONGWEI LIU [PHOTO COURTESY OF SHANSHAN QIN]](https://viterbischool.usc.edu/wp-content/uploads/2023/11/Longwei-Liu-Square-300x300.jpg)
Research Assistant Professor Longwei Liu. Image/SHANSHAN QIN
“Imagine if you wanted to find a very specific fish from the ocean — this would be really difficult,” Liu said. “But now with the directed evolution platform we developed, we do have a way to fish out these specific proteins with a desired function.”
The research team is now examining how to optimize the proteins further to make for even more precise and effective CAR T-cells before moving closer to clinical applications. This would also include integrating the proteins with the Wang Lab’s breakthrough applications of focused ultrasound to remotely control CAR T-cells so that they activate only at tumor sites.
“We now have all these genetic tools to manipulate, control and program those immune cells to have so much power and function,” Wang said. “We hope to create new ways to guide their function for particularly complicated solid tumor treatments. We are really excited about the possibilities.”
Published on April 22nd, 2024
Last updated on April 22nd, 2024